Mustache and Tail are my documents….
Distillation (Latin: distillatio – dripping) is the distillation, evaporation of a liquid, followed by cooling and condensation of vapors. The resulting condensate is called the distillate, and the unevaporated liquid is called the residue.
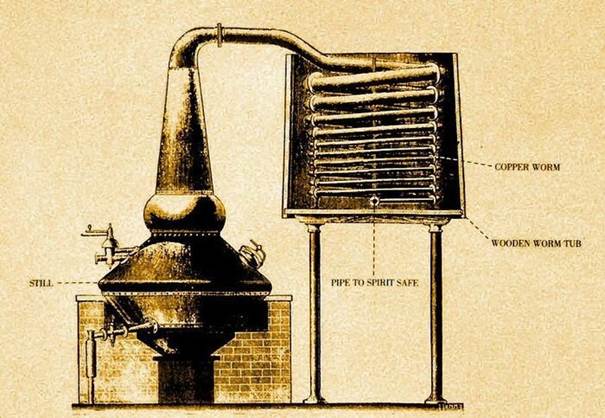
So, we have a liquid (mash) in a fermentation tank, which includes a large number of different components, including ethanol and ethyl alcohol. The method of distillation or rectification involves heating a liquid, as a result of which some components reach the boiling point and go into a vaporous state, this mass of suspended particles enters a condenser-cooler, where it is forcibly cooled and turns back into a liquid state.

The resulting mash is distilled two (sometimes three) times in copper stills (potstills), the shape of which vaguely resembles retorts.
As a result of distillation in the first washstill with a volume of 700-2300 decaliters, a liquid with a strength of 25-30% by volume is obtained, called “low wines”.
After the completion of the first distillation, the “weak wine” enters the second apparatus (spiritsstill), which has a volume of 600-2100 decaliters, and is distilled one more time.
The product of the second distillation is alcohol with a strength of up to 70% by volume. During the second distillation, the head and tail fractions are separated, i.e. those parts of the distillate that come out of the apparatus at the beginning and at the end of the distillation process, and only the middle fraction is selected (and this is only about 15% of the distillation).
Cubic residue up to 40%!
Distillation Technology
When the mash is ready, it is moved to the alembic, filling it to 1/2 – 2/3 of the volume, leaving room for the expansion of the mash and the foam formed during heating. The distillation time depends on the size and shape of the cube, the strength of the mash, etc. It continues until the strength of the contents in the distillation apparatus drops to 1%. The volume of the first distillation, the so-called “low wines”, is about a third of the original volume of mash and has a strength of 21 – 25 % by volume.
For the production of the second pasture, the strength of low wines must be at least 28%, and not more than 30%. Too weak will not be separated into fractions, and too strong contains undesirable substances (esters of higher fatty acids, etc.) that can reduce the quality of the future whiskey.

Lead Factions
These are volatile alcohols (having a low boiling point) that come out first at the start of distillation and include the following chemicals:
•Acetalhedide (CH3CHO) is an aldehyde produced by plants as part of their natural metabolic process. The same is obtained by the oxidation of ethanol. It has a boiling point of 20.8°C. It is also the main component that causes a hangover. It has a pungent fruity aroma reminiscent of the glandular notes of green apple.
• Acetone ((CH3)2CO) is a colorless, flammable liquid with a boiling point of 56.2°C. And if you feel these notes in alcohol, then acetone smells like them.
• Esters are naturally occurring chemical components that are responsible for the flavors of most fruits and berries, such as apples, pears, bananas, pineapples and strawberries. Basically, esters are formed by the condensation of carboxylic acids with alcohol. And their presence in the distillate determines the fruity notes. Esters are safe and have a sweet aroma. They are taken by those producers who want to get a lighter, fruity aroma, and finish the head fractions at a strength of 78-75%abv.
•Methanol (CH3OH, often abbreviated as MeOH) – also known as methyl alcohol, wood alcohol. It is a colorless, flammable liquid, with a boiling point of 64.7°C. Methanol and ethanol, like brother and sister, have a similar molecular structure, and close boiling points, so they are quite difficult to separate during the distillation process, but doable. The main thing to remember is that methanol is very harmful to the liver, and in large quantities it can lead to blindness (10 ml of methanol leads to blindness, and 30 ml can already have fatal consequences). All industrial whiskies produced by cube distillation can contain 4 to 5 parts per million of ethanol, and this level is considered safe. But about non-industrial ones is already a big question.
Heart
The heart is the main part that needs to be obtained. The main part is ethanol, but there may also be a small amount of head or tail components to give the desired character to the final alcohol.
• Ethanol (C2H5OH) – drinking alcohol. It is a colorless, flammable liquid. Due to its powerful effect on the central nervous system, leading to mood changes, it is the oldest legal drug.
In Scotland, heart is mainly taken in the range of 78%abv-58%abv, depending on the nature of the alcohols needed. If you need light and fruity, then the intake starts at 78%abv and ends up to 70%abv, but if you need heavier, oily, and especially peaty ones, then the intake starts at around 73%abv, and ends at 60%abv. At Springbank alone, up to 58% of abv is used to produce Longrow peat whisky.
Tails or faints
Chemical components that have a higher boiling point than ethanol and are obtained at the end of distillation.
• Propanol-1 (CH3CH2CH2OH) is formed in small quantities during fermentation and has a boiling point of 97.0°C. It is used as a solvent in pharmaceuticals, and is one of the alcohols related to fusel oils.
• Butanol or butyl alcohol (C4H10O) – formed during the fermentation of sugars, also present in beer and wine. It has a boiling point of 118°C, has a banana-like aroma, and belongs to fusel oils.
• Isoamyl alcohol is a colorless liquid with a boiling point of 131.6°C. It has a strong unpleasant aroma and a sharp burning taste. The same applies to fusel oils.
•Fusel oils is a general term for components found in tail fractions during distillation. These are high-order alcohols that have more than two carbon atoms and are highly soluble in water. They are obtained during the fermentation process and are present to varying degrees in beer, wine, cidre, mead and other beverages obtained during the fermentation process, as well as in distillation products from them. It has an oily consistency, which is why the name was obtained.
•Acetic acid (CH3COOH) is an organic acid formed during the fermentation process. It has a characteristic smell and sour taste. It dissolves well with water. The boiling point is 118.1°C.
• Furfural (OC4H3CHO) is an aromatic aldehyde derived from the bran of corn, oats and wheat. It is a colorless, oily liquid that quickly turns yellow when it comes into contact with oxygen. It has the smell of burnt and rotten nuts. It can also appear in cubes with direct heating as a result of burning. It has a boiling point of 161.7°C, but begins to evaporate much earlier.